Decontamination Management
Regardless of the automated decontamination system used, a proper implementation plan plays a pivotal role in reaching infection prevention goals. Any infection control or environmental service professional can attest to the complexity of this critical step. Bioquell offers comprehensive systems and services to achieve even aggressive infection rate reduction goals at any hospital. Whether you simply want a consult, or need to eliminate a recurring target pathogen like C. diff, Bioquell can help you lead the fight against HAIs.
Applications
Critical Care Units
Targeting Pathogen
and Reducing HAIs
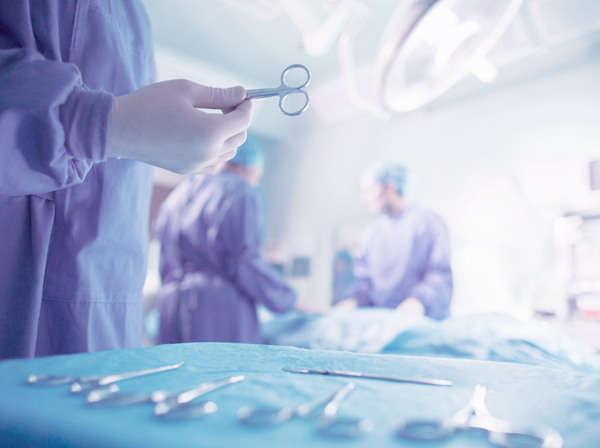
Operating Rooms
and Surgical Areas
Pre and Post Construction/Renovation
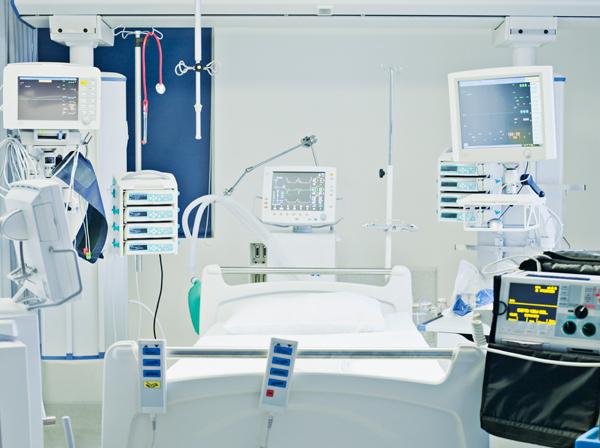
Critical Care Units
Critical care units require greater attention and focus on infection prevention. Because these patients are immunocompromised in this area, they have an increased risk for acquiring an infection. Our range of bio-decontamination solutions eliminate dangerous organisms in every nook and cranny, top to bottom, to stop pathogens from spreading from room to room or infecting the next occupant.
SYSTEMS AND SERVICES
Bioquell’s decontamination management solutions can help your facility even if you are not currently using Hydrogen Peroxide Vapor. Problem pathogens, outbreaks and emergency response situations for highly infectious diseases can be eliminated strategically and effectively.
Bioquell Proactive
The Bioquell Proactive provides a full-time Bioquell technician at your facility to perform daily decontamination with Hydrogen Peroxide Vapor in accordance with your infection control strategy. This service includes staffing, equipment needs and maintenance, consumables, reporting, technology upgrades and more.
Learn MoreRapid Bio-Decontamination Service (RBDS)
Infection control priorities can shift at any moment, requiring an advanced decontamination that UV and aerosolized technologies cannot provide. Bioquell’s Rapid Bio Decontamination Service (RBDS) can be quickly called upon to eliminate pathogens, stop outbreaks, handle emergency response situations and much more for any size area.
Learn MoreBioquell BQ-50
A mobile and robust hydrogen peroxide vapor generator, the Bioquell BQ-50 can be used hospital-wide to eliminate organisms from exposed surfaces. Get automated and proven results that can provide assistance in your infection control plans with Bioquell Hydrogen Peroxide Sterilant (EPA Reg. No. 72372-1-86703)
Learn MoreBioquell Pod
Creating single patient rooms from an open area in your hospital without absorbing construction costs or lengthy shutdown periods is all possible with the Bioquell Pod. This customizable solution is a quicker, cost effective alternative to permanent brick and mortar options. A single Bioquell Pod can help reduce HAIs, manage escalated incidents, provide patient privacy and more.
Learn MoreContact Us
To learn more about how Bioquell can fit your solution, please contact us.
The Americas
Ecolab Inc
702 Electronic Dr. Suite 200
Horsham, PA 19044
+1 215 682 0225
bioquellusorders@ecolab.com